Abstract
BACKGROUND: Neutrophils, the most abundant leukocytes and granulocytes, are important regulators of cardiovascular, inflammatory and infectious diseases, yet their role in the pathophysiology of clonal hematopoiesis of indeterminate potential (CHIP) has not been adequately addressed. The effects of inactivating CHIP-driver mutations in the epigenetic regulator TET2 in neutrophils especially, are broadly unknown.
HYPOTHESIS: Tet2 inactivation in murine neutrophils, and TET2 mutations in CHIP in humans (CHIP TET2), perturb granulocyte immune effector functions.
METHODS: Neutrophils were obtained (EasySep™, StemCell) from the bone marrow of 2- to 4-months-old, sex-matched, control Tet2 f/f;Vav1-icre - (Tet2 f/f) and hematopoietic knockout Tet2 f/f;Vav1-icre + (Tet2 -/-) mice. Neutrophils were cultured (RPMI+10% mouse serum/FBS) and: i) stained with Mitotracker Deep Red/Nuc Blue, co-cultured and imaged (Leica SP8-X) for 30min with GFP-labeled Staphylococcus aureus (10:1 ratio) and analyzed in FIJI; ii) cultured for 3h with vehicle or 10μg/mL of S. aureus lipotechoic acid (LTA). RNA-Seq was generated (Illumina QuantSeq 3' mRNA, single-end 75bp read lengths, 5 million reads/sample), trimmed, aligned to GRCm39 using STAR.
CHIP participant DNA and RNA were sequenced previously from whole blood (Cook et al., Bld Adv 2019; Cook et al., ASH 2018, with a 48-gene panel on Ion Proton, and ribo-depleted bulk RNA on Illumina, respectively). New CHIP TET2 vs. no CHIP, and murine RNA-Seq analyses were carried out in DESeq2. Human serum granule protein levels were quantified by ELISA (VersaMax).
Mann-Whitney U tests were carried out in Prism. P<0.05 was considered statistically significant, and Benjamini-Hochberg multiple testing correction was applied as needed.
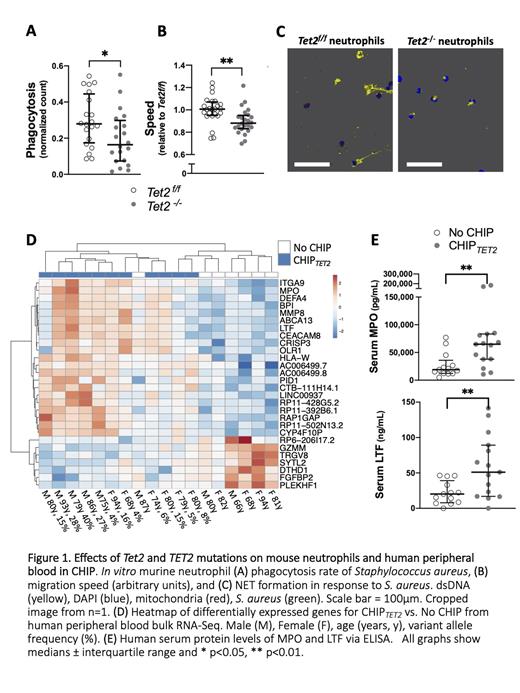
RESULTS: Tet2 -/- mice had 1.34-fold more bone marrow CD11b +Ly6G + neutrophils than control Tet2 f/f mice (p=0.03), consistent with myeloid expansion. Compared to Tet2 f/f, Tet2 -/- neutrophils phagocytosed fewer S. aureus (Fig1A) and moved more slowly (Fig1B). Preliminary data suggest that Tet2 -/- neutrophil extracellular trap (NET) formation in response to S. aureus was also impaired, showing fewer and less extensive NETs (Fig1C).
LTA-stimulated gene expression profiles were similar between Tet2 -/- and Tet2 f/f, suggesting pre-existing differences at baseline. Unexpectedly, the most significant GO term enrichment related to upregulated viral response pathways, including interferon-stimulated genes, (e.g. Ifitm1). The cause is unknown, but this is reminiscent of the constitutive interferon response seen in myelodysplastic syndrome (MDS) patients and TET2-mutant hematopoietic stem cells, where epigenetic dysregulation of endogenous retrotransposable elements leads to a viral mimicry response. Tet2 -/- neutrophils also overexpressed Asprv1, a regulator of inflammation ostensibly acquired from a retrotransposon. Interestingly, Ccdc80, which has been linked to Tet2 and Jak2 functions, was most significantly downregulated in Tet2 -/-, along with the Pnpla1 lipid phosphatase. Finally, Tesc, a promoter of granulocytic differentiation, was upregulated in Tet2 -/-, and there were perturbations of genes encoding neutrophil granule contents.
Similarly, human RNA-Seq revealed that several leukocyte (de)granulation-related genes (e.g. lactoferrin LTF, myeloperoxidase MPO) were upregulated in CHIP TET2 subjects to those without CHIP, and these corresponded with higher LTF and MPO serum titers in an expanded cohort (Fig1D,E). Finally, there were striking decreases of gene expression associated with cytotoxic (T/NK) human lymphocytes (i.e. GZMM, TRGV8, etc.). Neutrophil, lymphocyte and monocyte counts were not significantly different between the groups.
CONCLUSIONS: Tet2-deficient murine neutrophils have compromised immune function, possibly due to differences in pre-stimulus state. TET2-mutation carrying neutrophils in CHIP may exhibit similar abnormalities, as has been previously noted in neutrophils isolated from MDS patients. Indeed, CHIP is now known to associate with increased risk of bacterial and viral infections, and infection risk has also previously been noted for MDS. People with CHIP have elevated peripheral blood serum MPO and LTF levels, suggesting a difference in leukocyte granule biology, likely related to neutrophils. These data aid in understanding how CHIP alters immunity.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal